Comparison chart
| Low | High |
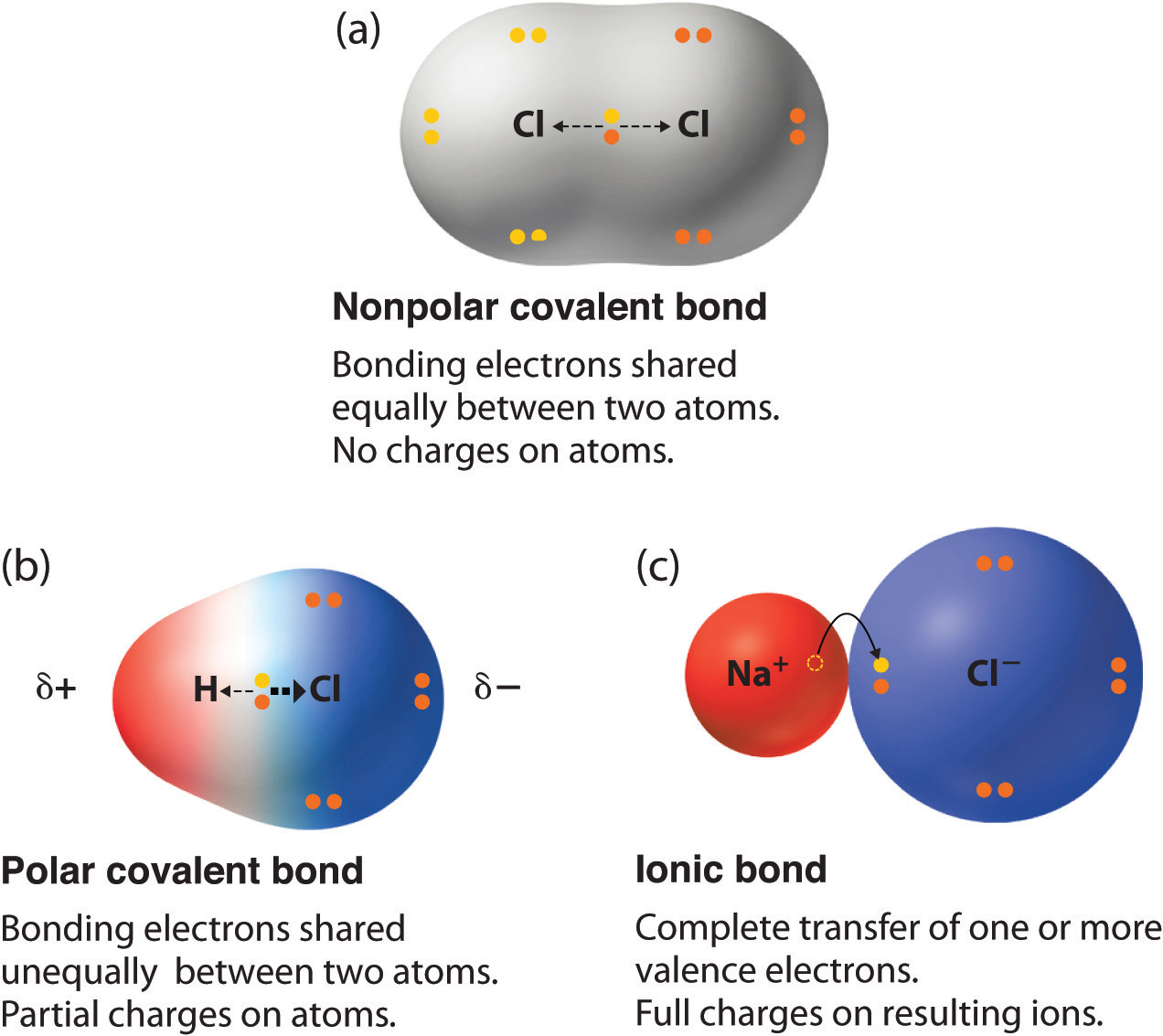
| A covalent bond is formed between two non-metals that have similar electronegativities. Neither atom is "strong" enough to attract electrons from the other. For stabilization, they share their electrons from outer molecular orbit with others | An ionic bond is formed between a metal and a non-metal. Non-metals(-ve ion) are "stronger" than the metal(+ve ion) and can get electrons very easily from the metal. These two opposite ions attract each other and form the ionic bond. |
| Definite shape | No definite shape |
| low | High |
| Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. | Ionic bond, also known as electrovalent bond is a type of bondformed from the electrostatic attraction between oppositely charged ions in a chemical compound. These kinds of bondsoccur mainly between a metallic and a non metallic atom. |
| Low | High |
| Methane (CH4), Hydro Chloric acid (HCl) | Sodium chloride (NaCl), Sulphuric Acid (H2SO4 ) |
| Two non-metals | One metal and one non-metal |
| Liquid or gaseous | Solid |
للمزيد :
ليست هناك تعليقات:
إرسال تعليق